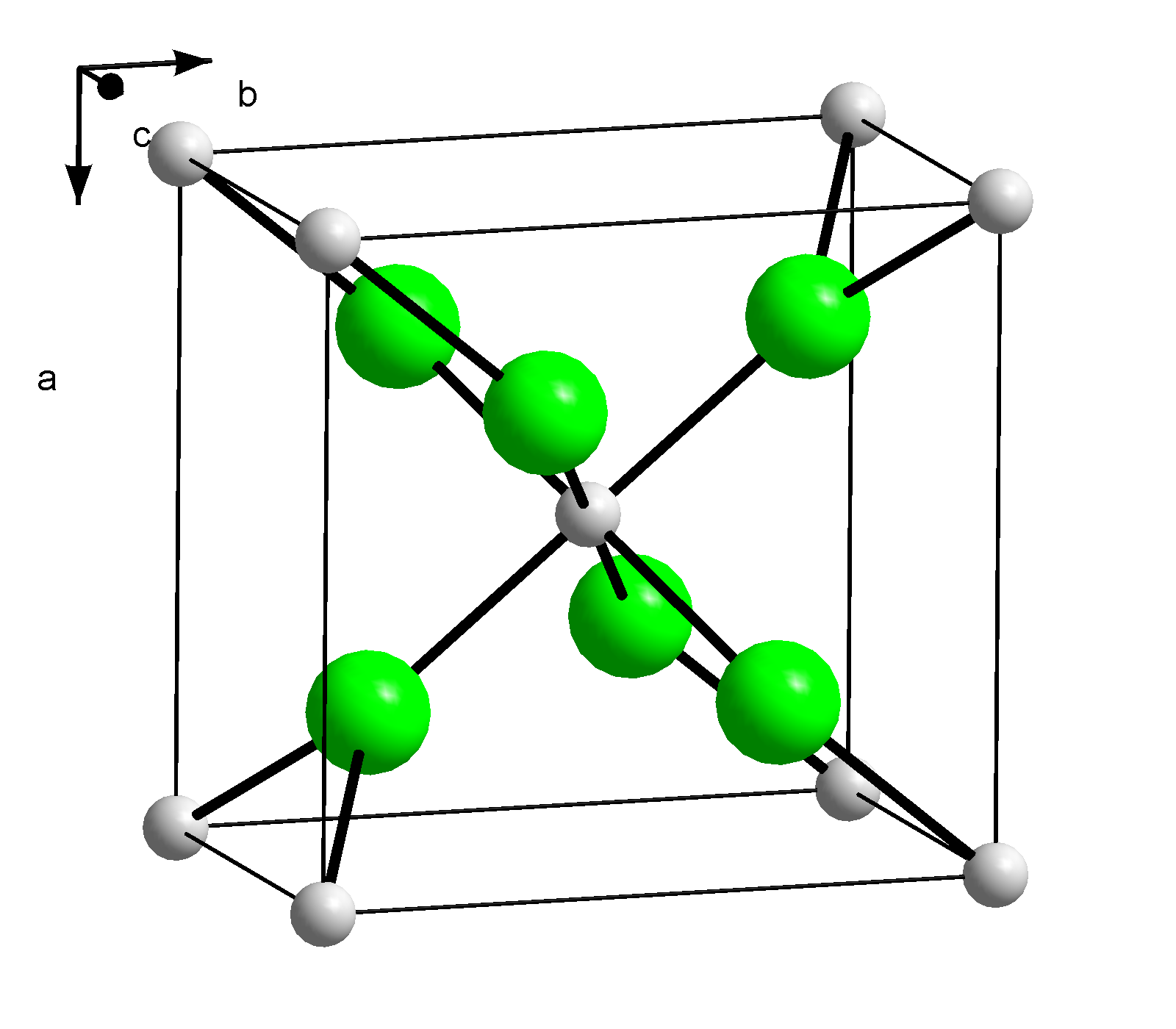
Question Video: Calculating the Mass of Calcium Chloride That Contains a Given Mass of Chlorine | Nagwa

Pyrohydrolysis of CaCl2 Waste for the Recovery of HCl Acid upon the Synergistic Effects from MgCl2 and Silica | ACS Sustainable Chemistry & Engineering

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

organic chemistry - What is role of copper powder, calcium chloride and cuprous chloride in the SN1 reaction of hydrochloric acid with propargylic alcohol? - Chemistry Stack Exchange
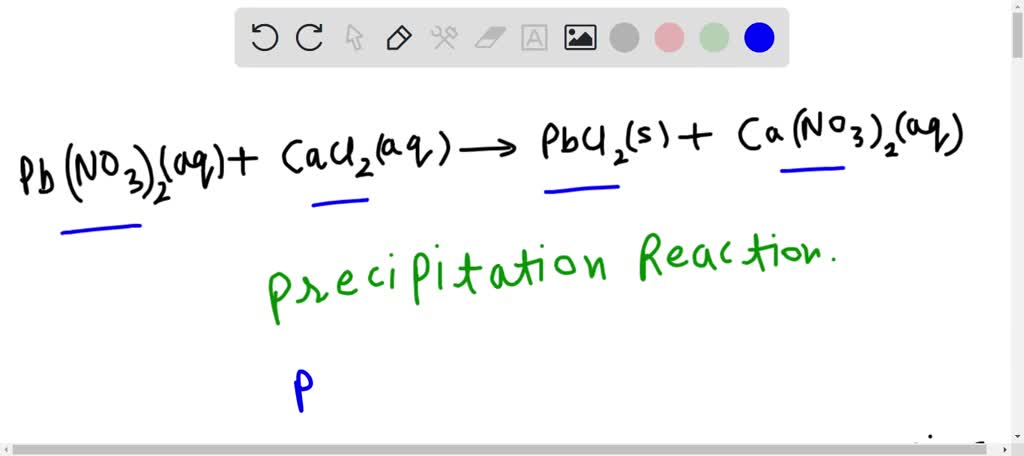
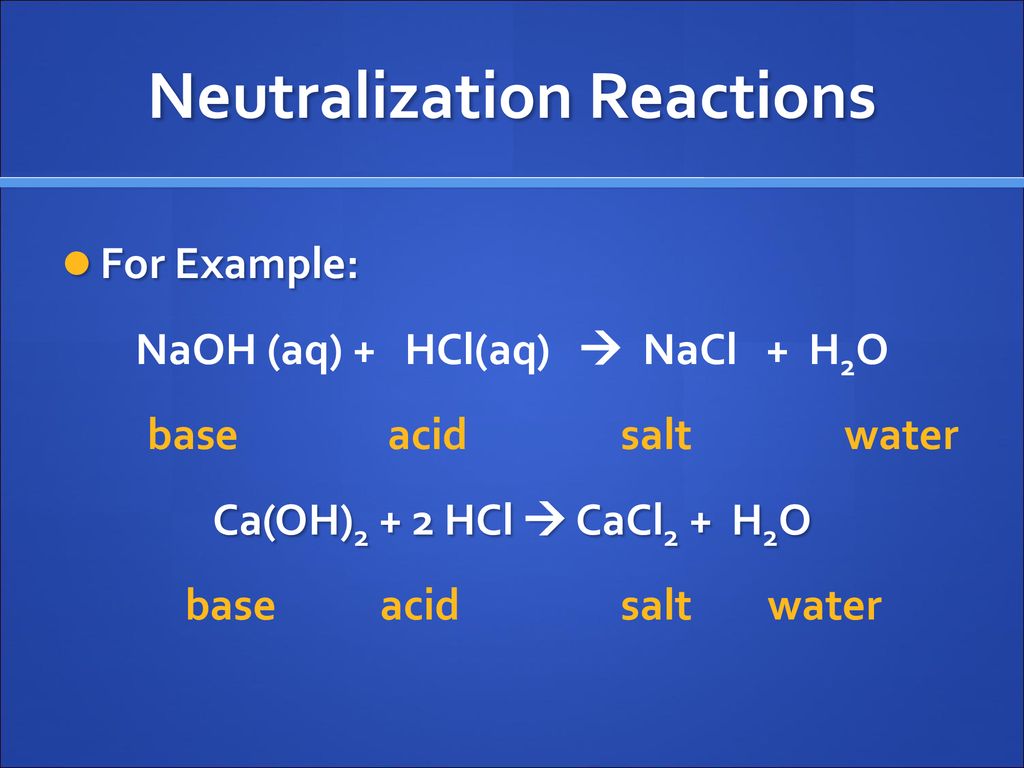
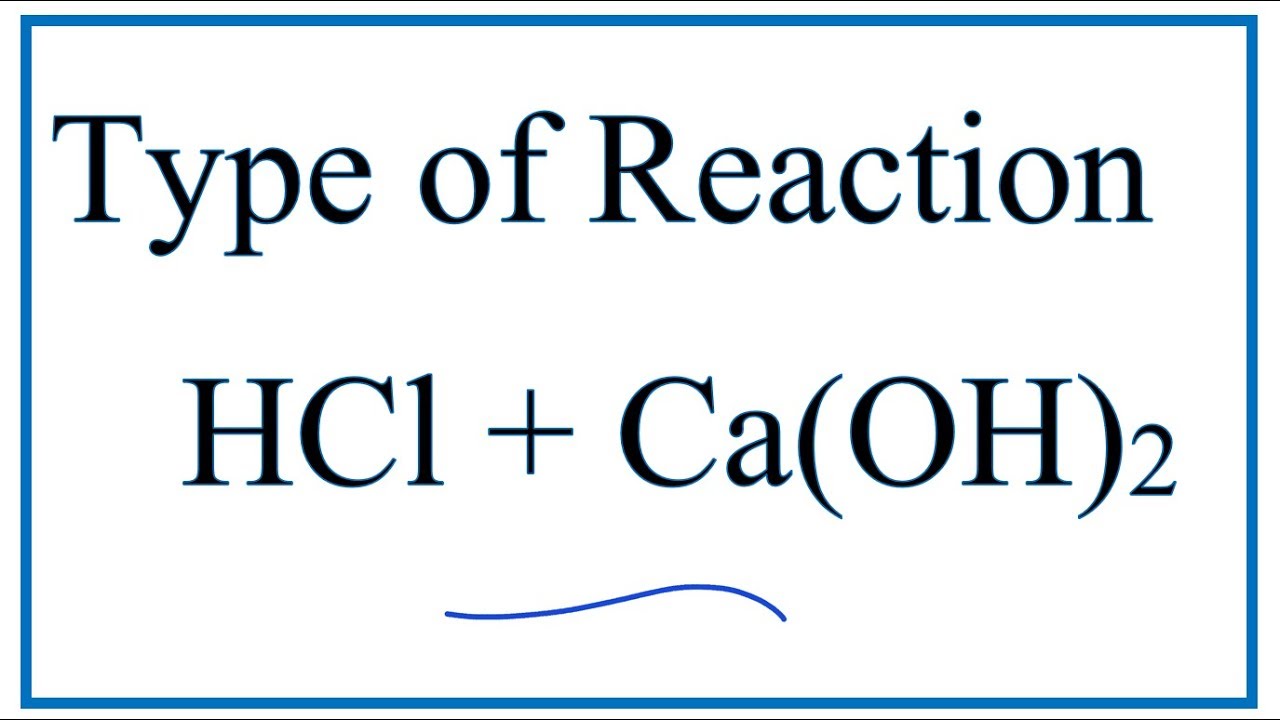
SOLVED: Choose the best classification of the reaction represented by the following equation: Pb(NO3)2(aq) + CaCl2(aq) → PbCl2(s) + Ca(NO3)2(aq) Multiple Choice decomposition acid-base oxidation-reduction neutralization precipitation





![HCl Gas from conc. HCl(aq) and CaCl2 - [www.rhodium.ws] HCl Gas from conc. HCl(aq) and CaCl2 - [www.rhodium.ws]](https://erowid.org/archive/rhodium/chemistry/equipment/pictures/calcium.hcl.gen.fig.gif)