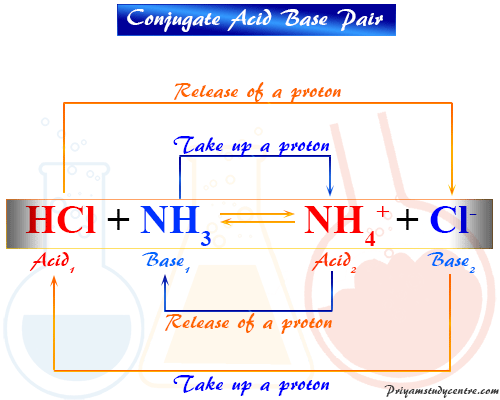
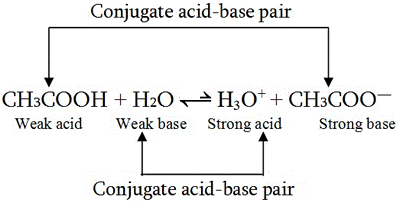
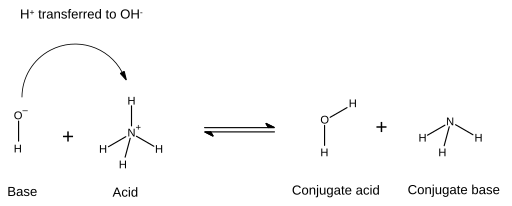
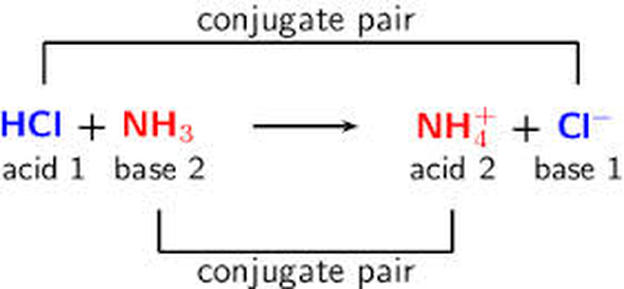
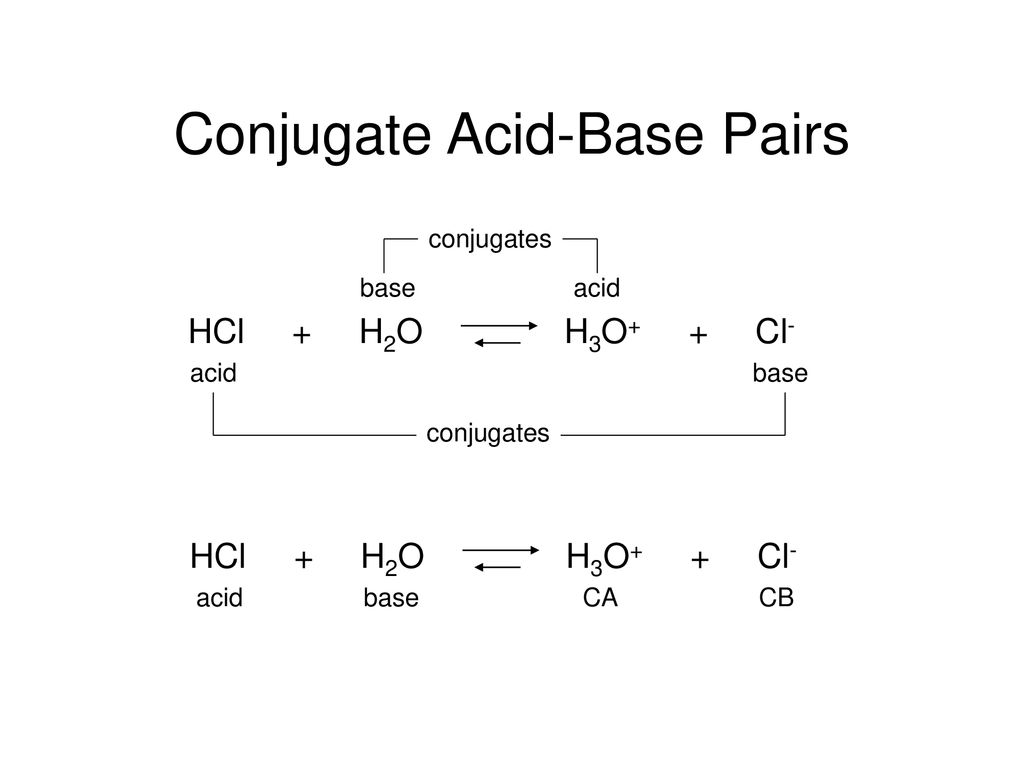
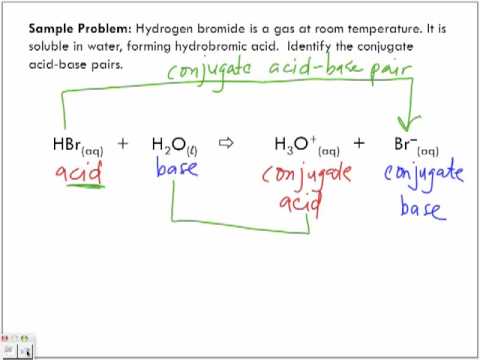
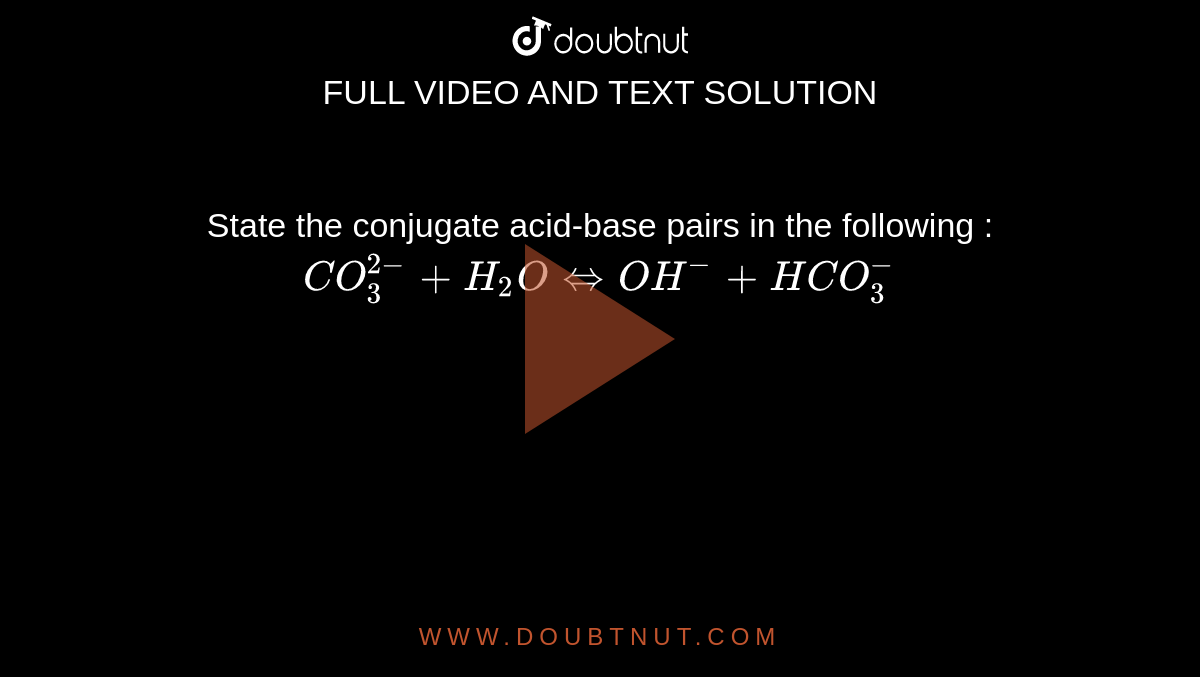
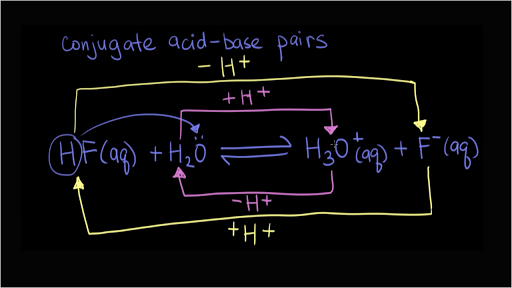
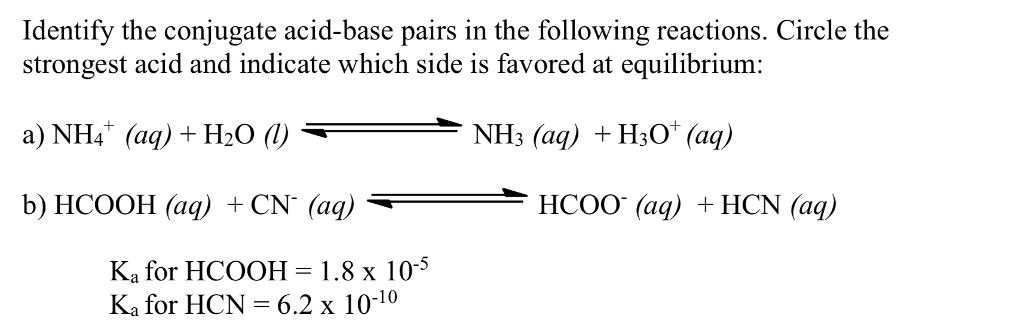Recap – Last Lecture An acid is a proton donor A base is a proton acceptor A conjugate pair differ by H + Strong A/B is completely dissociated Weak A/B. - ppt

SOLVED: Consider the reaction below. Which species are conjugate acid/base pairs? HSO:- (aq) HCN (aq) = HzSOz (aq) + CN- (aq) A) HSOs , CN- B) HSO: , HzSOz C) HzSO3, CN

Identify the conjugate acid-base pairs in the following reaction. Indicate what each substance is in each pair. H2CO3 + PO43- arrow HCO3- + HPO42- | Homework.Study.com

Relative strengths of some common conjugate acid- base pairs, listed... | Download Scientific Diagram
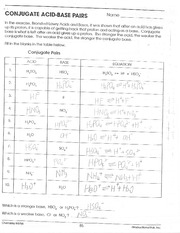
Conjugate Acid-Base Pairs - Fill in the blanks in the table Vbrelo'w.' Conjugate Pairs Which is a stronger base, HSO; or HZPOA'? ! '50“ Which is 0 | Course Hero

Identify the conjugate acid-base pairs for the reaction (with the acid written first). CN- + H2O = HCN + OH- |CN- / HCN |HCN / CN- |OH- / H2O |H2O / OH- | Homework.Study.com
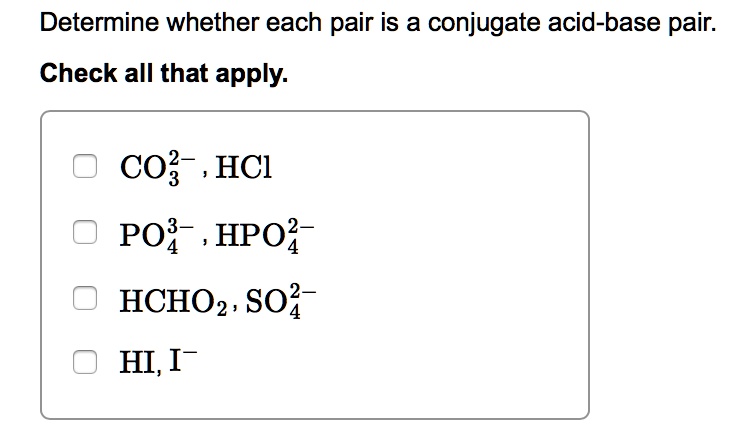
SOLVED: Determine whether each pair is a conjugate acid-base pair: Check all that apply: CO?- HCl PO? , HPO? HCHO2, S02 HI,I-